ITP’s Battery Test Centre
Six years and 26 batteries later
The residential battery energy storage market is young, but its consumers want products that will live to a ripe old age. In the beginning, there was only the caveated assurances of battery manufacturers. Now there is freely available, independent test data and reporting.

1 Nov 2022
Recognising the importance of residential battery storage in the Australian energy system, ARENA supported ITP Renewables’ Battery Test Centre with a $1.29m ARENA grant, under its Advancing Renewables Program. The testing under ARENA’s funding concluded in March 2022 and ITP Renewables is exploring other avenues of funding to continue testing.
The Battery Test Centre conducted longitudinal performance testing of conventional and emerging battery technologies with the aim of independently verifying claims made by manufacturers about the performance, installation, and operation of battery packs, and to disseminate the results to the public.
The Battery Test Centre facility, located at the Canberra Institute of Technology (CIT), is the only facility we are aware of to independently test a range of technologies and products over an extended period, and to publish results to the public.
Phase 1 of the trial started in mid-2016 and included eight batteries, with a further ten batteries installed as part of Phase 2 in mid-2017. In late 2019, an additional eight batteries were installed under Phase 3 of the trial.
ITP recently published their twelfth and final Public Report on the trial. The report describes testing results, general observations, and issues encountered throughout the trial with Phase 1, Phase 2, and Phase 3 batteries.
Previous reports are available here.
The batteries are mostly lithium-ion variants – either lithium iron phosphate or lithium nickel manganese cobalt chemistries. There are also a number of other chemistries including lithium titanate, lead-acid, lead-carbon, aqueous hybrid ion, zinc-bromine flow, and sodium nickel chloride technologies.
Testing procedure
Lab conditions mimicked real world, warm daytime and cool overnight temperatures. Each battery pack was charged over several hours, followed by a short rest period, then discharged over several hours, followed by another short rest period. In total, there were three charge/discharge cycles per day for most batteries (Note: Some batteries have restrictions on charge/discharge rates that reduce the number of cycles that can be completed each day). Originally the batteries were planned to be cycled at this rate for three years. However, additional funding from ARENA allowed batteries that were still operational to continue cycling beyond this point, with some in operation for over six years.
Time and throughput both degrade battery capacity. Our testing monitored this capacity fade, and other battery performance parameters, over each battery pack’s lifetime.
Key Findings
Battery Overview
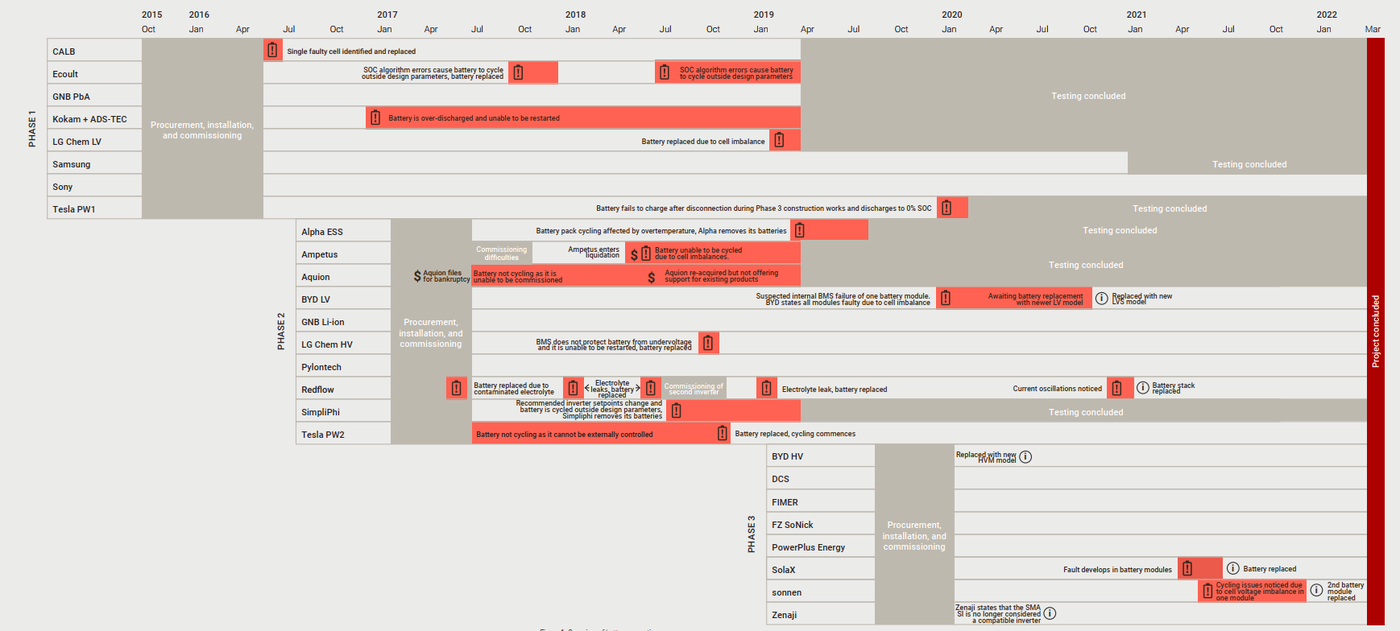
Below is a snapshot from the final report, showing the testing phase and status of all 26 batteries which were part of the trial.
Out of the eight Phase 1 batteries, the Sony battery pack ran continuously since its commissioning in 2016 and ITP continued to report its capacity fade results through to March 2022. The testing for the Samsung battery pack, which had also been in operation since 2016, concluded in early 2021.
Of the ten Phase 2 batteries, four concluded cycling before the end of the ARENA trial. Some of the remaining six were replaced during the testing period.
A further eight batteries were added in late 2019, and capacity fade trends started becoming apparent for the Phase 3 batteries which had done more than 500 cycles since installation.
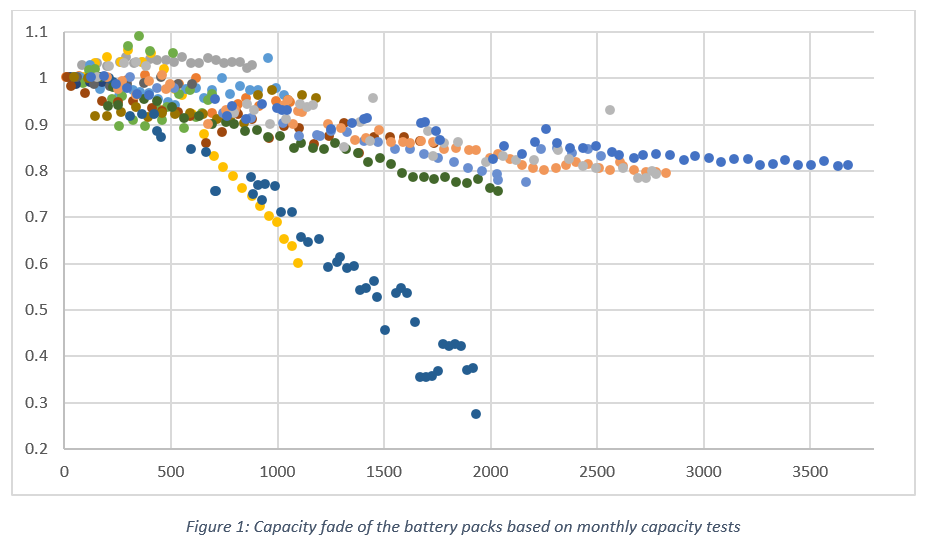
Capacity Fade
While some battery packs experienced faults and/or failed prematurely, the Sony, Samsung, BYD, GNB Lithium, Pylontech, and FIMER (ABB) battery packs generally demonstrated high reliability, with minimal issues encountered throughout the testing period.
Linear extrapolation of capacity fade suggests that cycle life varies significantly between products. The Sony and Pylontech battery packs demonstrated good capacity retention over a large number of cycles. Following replacements, the current FIMER, BYD HVM and Redflow ZCell also demonstrated excellent capacity retention, though the number of cycles completed was low when the final report was published.
ITP conducted monthly capacity tests for all batteries to compare the remaining capacity to its original capacity. This ratio is known as State of Health (SOH). Most of the batteries follow a similar general trend with their SOH, with just a few outliers as the graph shows.
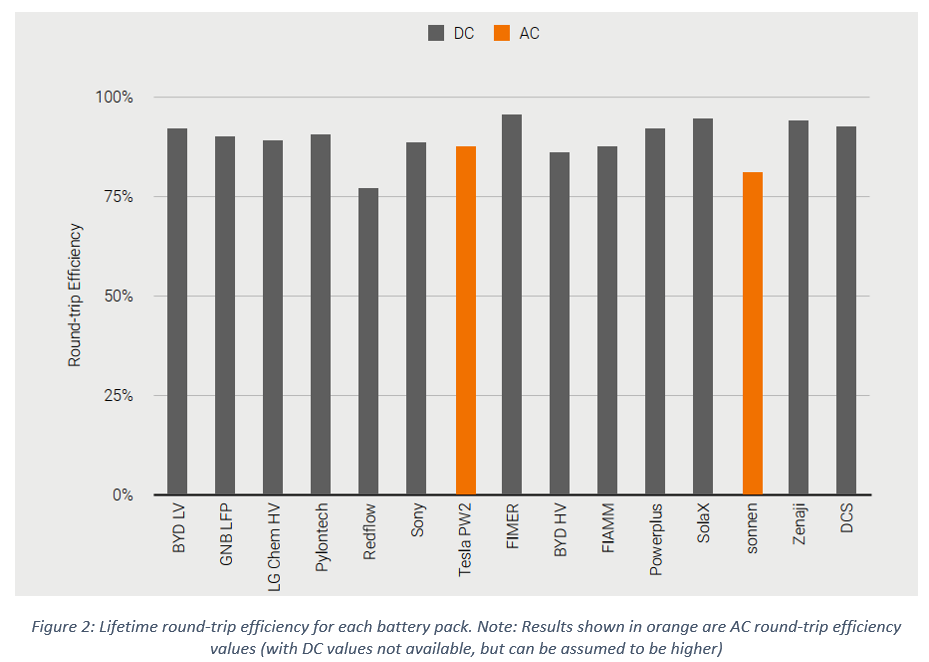
Round Trip Efficiency
Round-trip efficiency appeared more consistent between battery packs, with DC values as high as 95% for some lithium-ion battery packs, and as low as 78% for the zinc bromine ‘flow’ battery (which has more auxiliary loads such as pumps). The lifetime round-trip efficiency results are shown for each battery in the figure below.
Lessons Learned
Having been in operation for over six years, the Battery Test Centre revealed many valuable lessons, and greatly informed consumer decision making. The lessons learned relate not only to the performance of the batteries throughout the trial, but also to the performance of suppliers in delivering products and providing technical support during commissioning and operation.
The data published by ITP provided great value to the members of the public, as the test centre website acts as a resource for them before they purchase a battery. Lessons learned from the battery test centre are that:
- not all batteries are created equal, price does not guarantee quality,
- reliability is just as important as capacity retention, and
- product support and warranties should be considered alongside manufacturers’ performance specifications.
As the results are publicly available, we believe they have contributed to driving the battery storage industry towards an improvement in quality. Battery models have evolved and improved since the start of the testing. Many manufacturers now ensure that battery products, and their compatibility with specific inverter models, are tested before market release. Manufacturers are now moving towards requiring batteries to be directly connected to the internet and available for external monitoring, which allows them to remotely diagnose faults.
Increasingly, ITP is receiving inquiries from various battery manufacturers about the extension of the battery trial and being included for testing if the trial is extended. ITP also receives requests from international institutions for battery cycling data for use in energy storage modelling research. As more cycles are completed, the value of the information and longitudinal data about battery performance increases and ITP sees value in continuing the testing. As the industry develops, and until installations in the field have been operating for long enough, the Battery Test Centre’s results will continue to provide critical insights into the state of the battery storage market.